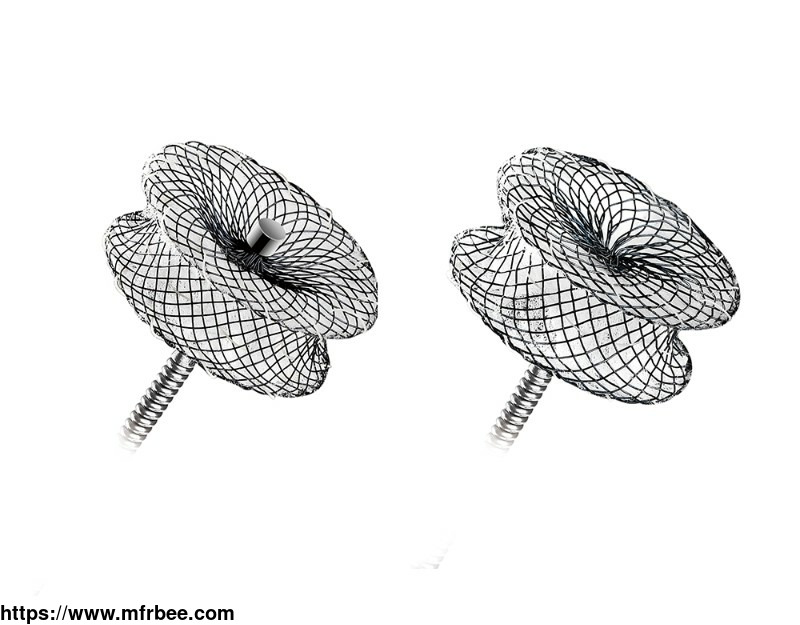
MemoPart™ Ventricular Septal Defect (VSD) Occluder
Specifications
MemoPart™
Fit to the clinic, long-term safety
MemoPart™ VSD Occluder Detail
Product Description
MemoPart™ Ventricular Septal Defect (VSD) Occluder is a self-expanding double-disc nitinol mesh occlusion device.The 2 discs are connected by a short waist which corresponds to the defect size. Polyester fabric is securely sewnto each disc to secure the occlusion. The device is visible under X-ray.
Indications for Use
Membranous VSD Occluder
The MemoPartTM Membranous VSD Occluder is used for minimally invasive transcatheter closure of perimembranous ventricular septal defects.
Muscular VSD Occluder
The MemoPartTM Muscular VSD Occluder is indicated for use in patients with a complex ventricular septal defect (VSD) of significant size to warrant closure (large volume left-to-right shunt, pulmonary hypertension,and/or clinical symptoms ofcongestive heart failure) who are considered to be at high risk for standard transatrial or transarterial surgical closure based on anatomical conditions and/or based on overall medical condition.
Ordering Information
Muscular Ventricular Septal Defect (VSD) Occluder
Catalogue No Device Size A Waist Diameter (mm) B
LV Disc
Diameter
(mm) C RV Disc Diameter (mm) H Waist Length (mm)
SQFDQ- Ⅰ a04 4 4 8 8 5.0
SQFDQ- Ⅰ a05 5 5 9 9 5.0
SQFDQ- Ⅰ a06 6 6 10 10 5.0
SQFDQ- Ⅰ a07 7 7 11 11 5.0
SQFDQ- Ⅰ a08 8 8 12 12 5.0
SQFDQ- Ⅰ a09 9 9 13 13 5.0
SQFDQ- Ⅰ a10 10 10 14 14 5.0
SQFDQ- Ⅰ a12 12 12 16 16 5.0
SQFDQ- Ⅰ a14 14 14 18 18 5.0
SQFDQ- Ⅰ a16 16 16 20 20 5.0
SQFDQ- Ⅰ a18 18 18 22 22 5.0
SQFDQ- Ⅰ b04 4 4 10 8 7.0
SQFDQ- Ⅰ b05 5 5 11 9 7.0
SQFDQ- Ⅰ b06 6 6 12 10 7.0
SQFDQ- Ⅰ b07 7 7 13 11 7.0
SQFDQ- Ⅰ b08 8 8 14 12 7.0
SQFDQ- Ⅰ b09 9 9 15 13 7.0
SQFDQ- Ⅰ b10 10 10 16 14 7.0
SQFDQ- Ⅰ b12 12 12 18 16 7.0
SQFDQ- Ⅰ b14 14 14 20 18 7.0
SQFDQ- Ⅰ b16 16 16 22 20 7.0
SQFDQ- Ⅰ b18 18 18 24 22 7.0
SQFDQ- Ⅰ c04 4 4 14 10 10
SQFDQ- Ⅰ c05 5 5 15 11 10
SQFDQ- Ⅰ c06 6 6 16 12 10
SQFDQ- Ⅰ c07 7 7 17 13 10
SQFDQ- Ⅰ c08 8 8 18 14 10
SQFDQ- Ⅰ c09 9 9 19 15 10
SQFDQ- Ⅰ c10 10 10 20 16 10
SQFDQ- Ⅰ c12 12 12 22 18 10
SQFDQ- Ⅰ c14 14 14 24 20 10
SQFDQ- Ⅰ c16 16 16 26 22 10
SQFDQ- Ⅰ c18 18 18 28 24 10
Hubless Muscular Ventricular Septal Defect (VSD) Occluder
Catalogue No Device Size A Waist Diameter (mm) B
LV Disc
Diameter
(mm) C RV Disc Diameter (mm) H Waist Length (mm)
WTSQFDQ- Ⅰ a04 4 4 8 8 5
WTSQFDQ- Ⅰ a05 5 5 9 9 5
WTSQFDQ- Ⅰ a06 6 6 10 10 5
WTSQFDQ- Ⅰ a07 7 7 11 11 5
WTSQFDQ- Ⅰ a08 8 8 12 12 5
WTSQFDQ- Ⅰ a09 9 9 13 13 5
WTSQFDQ- Ⅰ a10 10 10 14 14 5
WTSQFDQ- Ⅰ a12 12 12 16 16 5
WTSQFDQ- Ⅰ a14 14 14 18 18 5
WTSQFDQ- Ⅰ a16 16 16 20 20 5
WTSQFDQ- Ⅰ a18 18 18 22 22 5
WTSQFDQ- Ⅰ b04 4 4 10 8 7
WTSQFDQ- Ⅰ b05 5 5 11 9 7
WTSQFDQ- Ⅰ b06 6 6 12 10 7
WTSQFDQ- Ⅰ b07 7 7 13 11 7
WTSQFDQ- Ⅰ b08 8 8 14 12 7
WTSQFDQ- Ⅰ b09 9 9 15 13 7
WTSQFDQ- Ⅰ b10 10 10 16 14 7
WTSQFDQ- Ⅰ b12 12 12 18 16 7
WTSQFDQ- Ⅰ b14 14 14 20 18 7
WTSQFDQ- Ⅰ b16 16 16 22 20 7
WTSQFDQ- Ⅰ b18 18 18 24 22 7
WTSQFDQ- Ⅰ c04 4 4 14 10 10
WTSQFDQ- Ⅰ c05 5 5 15 11 10
WTSQFDQ- Ⅰ c06 6 6 16 12 10
WTSQFDQ- Ⅰ c07 7 7 17 13 10
WTSQFDQ- Ⅰ c08 8 8 18 14 10
WTSQFDQ- Ⅰ c09 9 9 19 15 10
WTSQFDQ- Ⅰ c10 10 10 20 16 10
WTSQFDQ- Ⅰ c12 12 12 22 18 10
WTSQFDQ- Ⅰ c14 14 14 24 20 10
WTSQFDQ- Ⅰ c16 16 16 26 22 10
WTSQFDQ- Ⅰ c18 18 18 28 24 10
Membranous Symmetric Ventricular Septal Defect (VSD) Occluder
Catalogue No. Device Size A
Waist
Diameter
(mm) B
LV Disc
Diameter
(mm) C RV Disc Diameter (mm) H
Waist
Length
(mm)
SQFDQ- Ⅱ b04 04 4 8 8 3.5
SQFDQ- Ⅱ b05 05 5 9 9 4.0
SQFDQ- Ⅱ b06 06 6 10 10 4.0
SQFDQ- Ⅱ b07 07 7 11 11 4.0
SQFDQ- Ⅱ b08 08 8 12 12 4.0
SQFDQ- Ⅱ b09 09 9 13 13 4.5
SQFDQ- Ⅱ b10 10 10 14 14 4.5
SQFDQ- Ⅱ b12 12 12 16 15 4.5
SQFDQ- Ⅱ b14 14 14 18 17 4.5
SQFDQ- Ⅱ b16 16 16 22 20 5.0
SQFDQ- Ⅱ b18 18 18 24 22 5.0
SQFDQ- Ⅱ b20 20 20 26 24 5.0
Hubless Membranous Symmetric Ventricular Septal Defect (VSD) Occluder
Catalogue No. Device Size A
Waist
Diameter
(mm) B
LV Disc
Diameter
(mm) C RV Disc Diameter (mm) H
Waist
Length
(mm)
WTSQFDQ- Ⅱ b04 04 4 8 8 3.5
WTSQFDQ- Ⅱ b05 05 5 9 9 4.0
WTSQFDQ- Ⅱ b06 06 6 10 10 4.0
WTSQFDQ- Ⅱ b07 07 7 11 11 4.0
WTSQFDQ- Ⅱ b08 08 8 12 12 4.0
WTSQFDQ- Ⅱ b09 09 9 13 13 4.5
WTSQFDQ- Ⅱ b10 10 10 14 14 4.5
WTSQFDQ- Ⅱ b12 12 12 16 15 4.5
WTSQFDQ- Ⅱ b14 14 14 18 17 4.5
WTSQFDQ- Ⅱ b16 16 16 22 20 5.0
WTSQFDQ- Ⅱ b18 18 18 24 22 5.0
WTSQFDQ- Ⅱ b20 20 20 26 24 5.0
Membranous Asymmetric Ventricular Septal Defect (VSD) Occluder for Multi-Fenestrated Defects
Catalogue No Device Size A Waist Diameter (mm) B LV Disc Diameter (mm) C RV Disc Diameter (mm) H Waist Length (mm)
SQFDQ- Ⅲ 04 04 4 12 8 3.5
SQFDQ- Ⅲ 05 05 5 13 9 4.0
SQFDQ- Ⅲ 06 06 6 14 10 4.0
SQFDQ- Ⅲ 07 07 7 15 11 4.0
SQFDQ- Ⅲ 08 08 8 16 12 4.0
SQFDQ- Ⅲ 09 09 9 17 13 4.5
SQFDQ- Ⅲ 10 10 10 18 14 4.5
SQFDQ- Ⅲ 12 12 12 20 16 4.5
SQFDQ- Ⅲ 14 14 14 22 18 4.5
SQFDQ- Ⅲ 16 16 16 24 20 5.0
SQFDQ- Ⅲ 18 18 18 26 22 5.0
Hubless Membranous Asymmetric Ventricular Septal Defect (VSD) Occluder for Multi-Fenestrated Defects
Catalogue No Device Size A Waist Diameter (mm) B LV Disc Diameter (mm) C RV Disc Diameter (mm) H Waist Length (mm)
WTSQFDQ- Ⅲ 04 04 4 12 8 3.5
WTSQFDQ- Ⅲ 05 05 5 13 9 4.0
WTSQFDQ- Ⅲ 06 06 6 14 10 4.0
WTSQFDQ- Ⅲ 07 07 7 15 11 4.0
WTSQFDQ- Ⅲ 08 08 8 16 12 4.0
WTSQFDQ- Ⅲ 09 09 9 17 13 4.5
WTSQFDQ- Ⅲ 10 10 10 18 14 4.5
WTSQFDQ- Ⅲ 12 12 12 20 16 4.5
WTSQFDQ- Ⅲ 14 14 14 22 18 4.5
WTSQFDQ- Ⅲ 16 16 16 24 20 5.0
WTSQFDQ- Ⅲ 18 18 18 26 22 5.0
Zero Rim Eccentric Ventricular Septal Defect (VSD) Occluder for the Defect Close to Aortic Valve
Catalogue No Device Size A Waist Diameter (mm) B LV Disc Diameter (mm) C RV Disc Diameter (mm) H Waist Length (mm)
SQFDQ- Ⅳ04 04 4 9 8 3.5
SQFDQ- Ⅳ 05 05 5 10 9 3.5
SQFDQ- Ⅳ 06 06 6 11 10 4.0
SQFDQ- Ⅳ 07 07 7 12 11 4.0
SQFDQ- Ⅳ 08 08 8 13 12 4.5
SQFDQ- Ⅳ 09 09 9 14 13 5.0
SQFDQ- Ⅳ 10 10 10 17 15 5.0
SQFDQ- Ⅳ 12 12 12 20 18 5.0
SQFDQ- Ⅳ 14 14 14 22 20 5.0
SQFDQ- Ⅳ 16 16 16 24 22 5.0
To build Lepu Medical into a large-scale global company in the cardiovascular industry that provides products and solutions covering prevention, treatment and rehabilitation for the patients with cardiovascular and related diseases.
If you want to know more kinds of medical devices china, please visit our website.
- Country: China (Mainland)
- Address: No.37 Chaoqian Road Changping District, Beijing, China
- Contact: lepumedical